Unveiled: The Untold Truth of Autoimmune Frontiers
Globally, autoimmune diseases(ADs)have emerged as the second-largest therapeutic area, trailing only oncology. In 2022, the global autoimmune disease drug market reached132.3billion,projectedtosurgeto176.7 billion by 2030, with biologics dominating 82.1% of the market. As evidenced by the American Autoimmune Related Diseases Association’s disease registry, the human body harbors over 150 distinct autoimmune conditions.

Beyond atopic dermatitis, lupus erythematosus, psoriasis, and myasthenia gravis, there exist numerous other subtypes of autoimmune diseases!
Classified by the scope of affected organs, these conditions fall into two categories: organ-specificADsand systemicADs. The former manifests as localized lesions confined to specific organs, with common examples including Hashimoto's thyroiditis, Graves' disease, inflammatory bowel disease, and celiac disease.
The latter involves multi-organ involvement affecting systems such as skin, joints, muscles, blood, kidneys, and nervous systems. Representative examples encompass rheumatoid arthritis, Sjögren's syndromeandsystemic sclerosis.
Autoimmune Disease Research Landscape:
Multidimensional Challenges Require Robust Randomized Methodologies
The global incidence of autoimmune diseases continues to rise, driven by genetic predispositions, environmental triggers, infectious agents, and evolving lifestyle patterns. Concurrently, heightened societal awareness enables earlier diagnosis and personalized therapeutic interventions, delivering enhanced clinical outcomes for patients.
However, numerous rare autoimmune disorders—including pemphigus, Behçet's disease, and Sjögren's syndrome—persist as therapeutic deserts. Their development pipelines face formidable barriers: high-risk drug discovery paradigms, intricate clinical trial designs, and constrained market incentives.
1.The Challenge
Autoimmune diseases inherently exhibithigh heterogeneity, with significant inter-subject response variability, necessitatingsophisticated stratified randomization strategiesto ensure equitable allocation across patient subtypes. The potential requirement forre-randomizationarises as subjects' conditions evolve during studies, particularly in cases of slow disease progression or fluctuating symptomatology. Additionally,ensuring scientific rigor in randomization protocolsremains an operational challenge in small-sample autoimmune trials.
2.The Solution
A highly flexible and scalable randomization system is paramount, demanding rigorous evaluation of its capacity to deliver stratified randomization, re-randomization, and dynamic allocation algorithms with proven efficacy.Robust audit trail mechanismscanensure allocation equity while minimizing human error.When selecting vendors, prioritizing those offering functionally comprehensive yet intuitively operable platforms becomes imperative to ensure frictionless trial lifecycle management.
OurRandomization & Drug Management System, ACCMED-IRT,supports fully customizable workflows for clinical trial design, randomization algorithms, and subject management, ensuring 100% reproducible randomization lists, tiered operational permissions, and configurable subject activation/lockout timelines to mitigate study risks. Its versatile drug supply strategies streamline end-to-end deployment from design to execution, enabling precise application across complex clinical scenarios.
Autoimmune diseases represent a blue-ocean market frontier, driven both by their intrinsic clinical complexity and China's accelerating regulatory tailwinds supporting drug innovation and reimbursement policy evolution. With autoimmune therapeutics becoming thestrategic imperativefor pharma innovators, aligning withaudit-proven, expertise-driven clinical research partnersemerges as the catalyst to transform trial execution from burdensome to breakthrough.
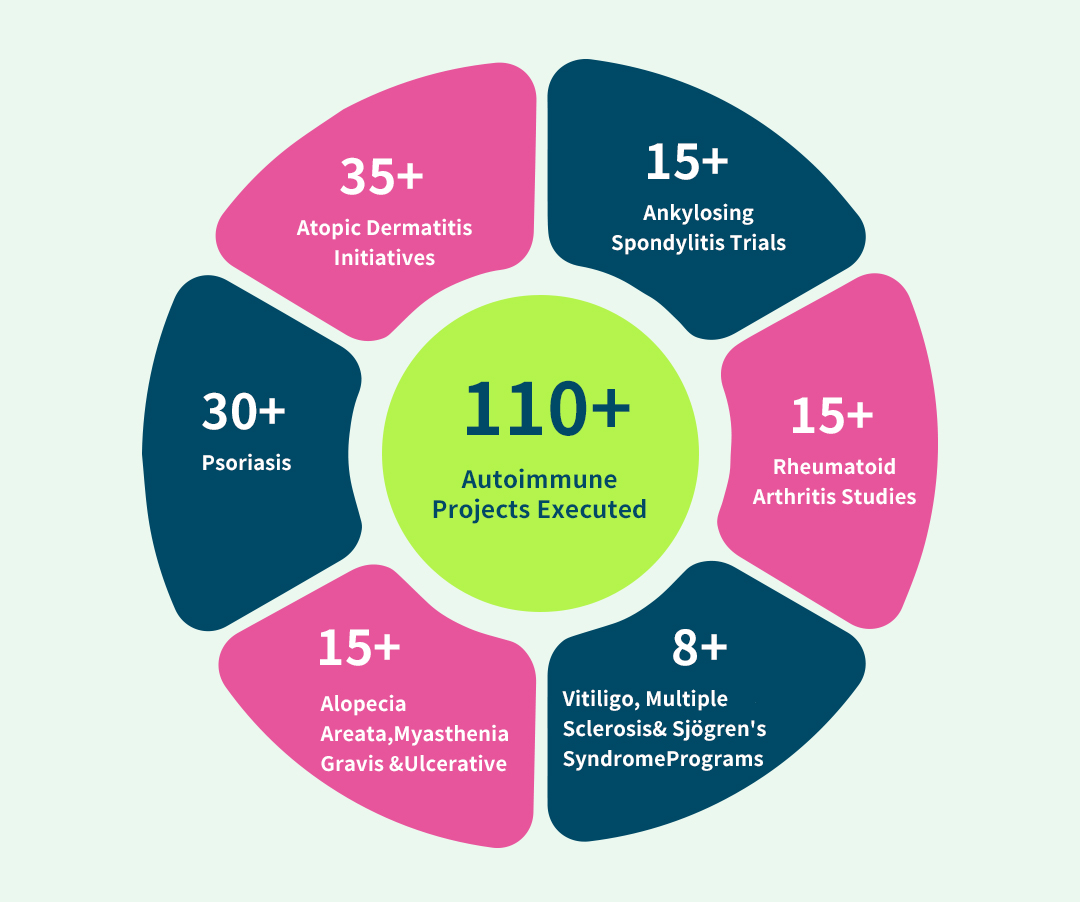
With 110+ cumulative autoimmune projectsspanning atopic dermatitis, psoriasis, rheumatoid arthritis, ankylosing spondylitis, alopecia areata, myasthenia gravis, ulcerative colitis, multiple sclerosis, and Sjögren's syndrome — whether mainstream or niche indications — we deliverprotocol-optimized solutionsthrough unparalleled therapeutic area mastery.
Furthermore, our operations adhere toGCP gold standards, seamlessly aligning withglobal compliance frameworks encompassingNMPA, FDA, EMA, TGA, and PMDAmandates, validated through60+ successful regulatory audits.
Partner withACCMEDfor AutoimmuneDisease Research
Optimize Drug Supply Chains with Proven Strategies
Achieve Full-Cycle Precisionin Clinical Operations
Resolve Clinical Research Challenges with Unmatched Ease